5’-Fluorescein phosphoramidite
基本信息
| 产品名称 | 5’-Fluorescein phosphoramidite |
|---|---|
| 英文名称 | 5’-Fluorescein phosphoramidite |
一般描述
产品描述
Ex(nm):494
Em(nm):522
Solvent:MeCN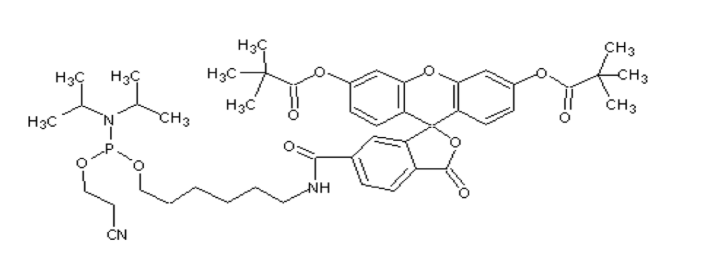
There are several ways of labeling an oligonucleotide with fluorescein. The choice of label is diversified further, depending on the spectral requirements. 5’-fluorescein-CE phosphoramidite, derived from the single isomer 6-carboxyfluorescein, 5’-hexachlorofluorescein-CE phosphoramidite (HEX) and 5’-tetrachlorofluorescein-CE phosphoramidite (TET) can all be used to efficiently label an oligonucleotide at the 5’-end. Labeling the 3’-end of an oligo with fluorescein can be achieved using 3’-Fluorescein CPGs. Standard cleavage and deprotection with ammonium hydroxide liberates the fluorescein-labeled oligo when using any of these supports.
参考文献
1. Browne KA. (2005) Sequence-specific, self-reporting hairpin inversion probes. J Am Chem Soc, 127, 1989.
2.Dobson N, McDowell DG, French DJ, Brown LJ, Mellor JM, Brown T. (2003) Synthesis of HyBeacons and dual-labelled probes containing 2′-fluorescent groups for use in genetic analysis. Chem Commun (Camb), 1234.
3. Hagmar P, Bailey M, Tong G, Haralambidis J, Sawyer WH, Davidson BE. (1995) Synthesis and characterisation of fluorescent oligonucleotides. Effect of internal labelling on protein recognition. Biochim Biophys Acta, 1244, 259.
4. Hakala H, Virta P, Salo H, Lonnberg H. (1998) Simultaneous detection of several oligonucleotides by time-resolved fluorometry: the use of a mixture of categorized microparticles in a sandwich type mixed-phase hybridization assay. Nucleic Acids Res, 26, 5581.
5. Hill KW, Taunton-Rigby J, Carter JD, Kropp E, Vagle K, Pieken W, McGee DP, Husar GM, Leuck M, Anziano DJ, Sebesta DP. (2001) Diels–Alder bioconjugation of diene-modified oligonucleotides. J Org Chem, 66, 5352.
6. Kim SJ, Bang EK, Kwon HJ, Shim JS, Kim BH. (2004) Modified oligonucleotides containing lithocholic acid in their backbones: their enhanced cellular uptake and their mimicking of hairpin structures. Chembiochem, 5, 1517.
7. Kutyavin IV, Lokhov SG, Afonina IA, Dempcy R, Gall AA, Gorn VV, Lukhtanov E, Metcalf M, Mills A, Reed MW, Sanders S, Shishkina I, Vermeulen NM. (2002) Reduced aggregation and improved specificity of G-rich oligodeoxyribonucleotides containing pyrazolo[3,4-d]pyrimidine guanine bases. Nucleic Acids Res, 30, 4952.
8. Lee SP, Censullo ML, Kim HG, Knutson JR, Han MK. (1995) Characterization of endonucleolytic activity of HIV-1 integrase using a fluorogenic substrate. Anal Biochem, 227, 295.
9. Meyer KL, Hanna MM. (1996) Synthesis and characterization of a new 5-thiol-protected deoxyuridine phosphoramidite for site-specific modification of DNA. Bioconjug Chem, 7, 401.
Product description
Ex(nm):494
Em(nm):522
Solvent:MeCN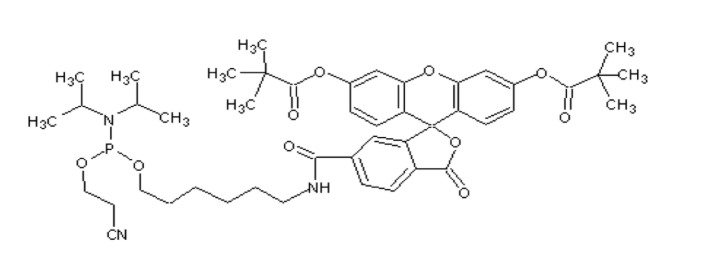
Features and Biological Applications
There are several ways of labeling an oligonucleotide with fluorescein. The choice of label is diversified further, depending on the spectral requirements. 5’-fluorescein-CE phosphoramidite, derived from the single isomer 6-carboxyfluorescein, 5’-hexachlorofluorescein-CE phosphoramidite (HEX) and 5’-tetrachlorofluorescein-CE phosphoramidite (TET) can all be used to efficiently label an oligonucleotide at the 5’-end. Labeling the 3’-end of an oligo with fluorescein can be achieved using 3’-Fluorescein CPGs. Standard cleavage and deprotection with ammonium hydroxide liberates the fluorescein-labeled oligo when using any of these supports.
References
1. Browne KA. (2005) Sequence-specific, self-reporting hairpin inversion probes. J Am Chem Soc, 127, 1989.
2.Dobson N, McDowell DG, French DJ, Brown LJ, Mellor JM, Brown T. (2003) Synthesis of HyBeacons and dual-labelled probes containing 2′-fluorescent groups for use in genetic analysis. Chem Commun (Camb), 1234.
3. Hagmar P, Bailey M, Tong G, Haralambidis J, Sawyer WH, Davidson BE. (1995) Synthesis and characterisation of fluorescent oligonucleotides. Effect of internal labelling on protein recognition. Biochim Biophys Acta, 1244, 259.
4. Hakala H, Virta P, Salo H, Lonnberg H. (1998) Simultaneous detection of several oligonucleotides by time-resolved fluorometry: the use of a mixture of categorized microparticles in a sandwich type mixed-phase hybridization assay. Nucleic Acids Res, 26, 5581.
5. Hill KW, Taunton-Rigby J, Carter JD, Kropp E, Vagle K, Pieken W, McGee DP, Husar GM, Leuck M, Anziano DJ, Sebesta DP. (2001) Diels–Alder bioconjugation of diene-modified oligonucleotides. J Org Chem, 66, 5352.
6. Kim SJ, Bang EK, Kwon HJ, Shim JS, Kim BH. (2004) Modified oligonucleotides containing lithocholic acid in their backbones: their enhanced cellular uptake and their mimicking of hairpin structures. Chembiochem, 5, 1517.
7. Kutyavin IV, Lokhov SG, Afonina IA, Dempcy R, Gall AA, Gorn VV, Lukhtanov E, Metcalf M, Mills A, Reed MW, Sanders S, Shishkina I, Vermeulen NM. (2002) Reduced aggregation and improved specificity of G-rich oligodeoxyribonucleotides containing pyrazolo[3,4-d]pyrimidine guanine bases. Nucleic Acids Res, 30, 4952.
8. Lee SP, Censullo ML, Kim HG, Knutson JR, Han MK. (1995) Characterization of endonucleolytic activity of HIV-1 integrase using a fluorogenic substrate. Anal Biochem, 227, 295.
9. Meyer KL, Hanna MM. (1996) Synthesis and characterization of a new 5-thiol-protected deoxyuridine phosphoramidite for site-specific modification of DNA. Bioconjug Chem, 7, 401.
相关属性
| CAS编号 | 204697-37-0 |
|---|---|
| 分子量 | 843.94 |
| 分子式 | C46H58N3O10P |
| 品牌 | Jinpan |
| PubChem CID | 58114954 |