6-TAMRA CPG *1000 Å*
基本信息
| 产品名称 | 6-TAMRA CPG *1000 Å* |
|---|---|
| 英文名称 | 6-TAMRA CPG *1000 Å* |
| 运输条件 | 超低温冰袋运输 |
一般描述
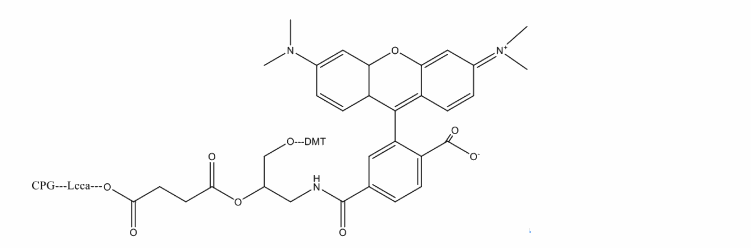
The light-absorbing properties of TAMRA, and spectral overlap with several commonly used fluorophores – including FAM, HEX, TET and JOE, make it useful as a FRET acceptor for the dual labeled FRET probes such as Molecular Beacons. TAMRA has been used extensively for real-time PCR and other molecular diagnostic applications. Oligonucleotides can be labeled with TAMRA using two distinct methodologies. Under standard deprotection conditions, TAMRA is not sufficiently stable; the molecule degrades in the presence of ammonium hydroxide. If standard deprotection is required, the oligonucleotide is normally synthesized with an amino group at either the 3′-, or 5′-end and post-labeled with TAMRA succinimidyl ester. Oligonucleotides synthesized using UltraMILD monomers can also be labeled directly with TAMRA, either internally by substituting any suitable dT residue with TAMRA-dT-CE phosphoramidite, or at the 3′-end using 3′-TAMRA CPG support. Subsequent deprotection of the oligo using potassium carbonate in methanol adequately removes the base protecting groups, leaving the TAMRA intact. Alternatively the deprotection of 1:1:2 t-butylamine/methanol/water allows the use of regular monomers.
Ex(nm):552
Em(nm):578
Solvent:MeCN
Chemical Structure:
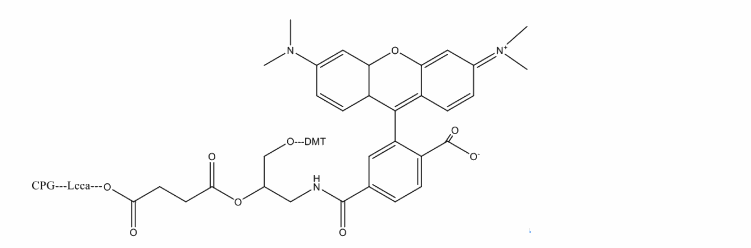
The light-absorbing properties of TAMRA, and spectral overlap with several commonly used fluorophores – including FAM, HEX, TET and JOE, make it useful as a FRET acceptor for the dual labeled FRET probes such as Molecular Beacons. TAMRA has been used extensively for real-time PCR and other molecular diagnostic applications. Oligonucleotides can be labeled with TAMRA using two distinct methodologies. Under standard deprotection conditions, TAMRA is not sufficiently stable; the molecule degrades in the presence of ammonium hydroxide. If standard deprotection is required, the oligonucleotide is normally synthesized with an amino group at either the 3′-, or 5′-end and post-labeled with TAMRA succinimidyl ester. Oligonucleotides synthesized using UltraMILD monomers can also be labeled directly with TAMRA, either internally by substituting any suitable dT residue with TAMRA-dT-CE phosphoramidite, or at the 3′-end using 3′-TAMRA CPG support. Subsequent deprotection of the oligo using potassium carbonate in methanol adequately removes the base protecting groups, leaving the TAMRA intact. Alternatively the deprotection of 1:1:2 t-butylamine/methanol/water allows the use of regular monomers.
Ex(nm):552
Em(nm):578
Solvent:MeCN
Chemical Structure:
相关属性
| 敏感性 | 对光和湿度敏感 |
|---|---|
| 储存温度 | 避光,-20°C储存,干燥 |
| 品牌 | Jinpan |