产品组成
|
|
产品名称
|
包装
|
|
A液
|
H2DCFH-DA(10mM)
|
0.1mL
|
|
B液
|
活性氧阳性对照(Rosup,50mg/mL)
|
1mL
|
产品简介
活性氧检测试剂盒(Reactive Oxygen Species Assay Kit)是一种利用荧光探针H2DCFH-DA进行活性氧检测的试剂盒。H2DCFH-DA本身没有荧光,可以自由穿过细胞膜,进入细胞内后,可以被细胞内的酯酶水解生成DCFH。而DCFH不能通透细胞膜,从而使探针很容易被装载到细胞内。细胞内的活性氧可以氧化无荧光的DCFH生成有荧光的DCF。检测DCF的荧光就可以知道细胞内活性氧的水平。根据活细胞中荧光的产生,可以判断细胞活性氧的含量和变化。用流式细胞仪或荧光显微镜可直接观察,是一种经典的组织或活细胞中活性氧检测方法。本试剂盒提供了活性氧阳性对照试剂Rosup,以便于活性氧的检测。Rosup是一种混合物,浓度为50mg/mL。Rosup 为活性氧阳性诱导药物,根据其荧光信号强度,可分析活性氧的真正水平。
本试剂盒本底低,灵敏度高,线性范围宽,使用方便。本试剂盒可以测定1000个样品1000T(96 孔板)。
一套包含96孔板,可使用1000次。
对光敏感,注意避光保存
注意事项
1. 探针装载后,一定要洗净残余的未进入细胞内的探针,否则会导致背景较高。
2. 阳性对照Rosup 一般使用浓度为100 μM (推荐浓度100-400μM,具体依细胞类型而定)。通常刺激后0.5-4h 可观察到显著的活性氧水平升高。对于不同的细胞,活性氧阳性对照的效果可能有较大的差别。如果在刺激后30min 内观察不到活性氧的升高,可延长诱导时间或适当提高活性氧阳性对照的浓度。如果活性氧升高得过快,可缩短诱导时间或适当降低活性氧阳性对照的浓度。
3. 对于某些特别的细胞,实验过程中如果发现没有刺激的阴性对照细胞荧光也比较强, 可以按照1:2000-1:5000 稀释DCFH-DA , 使DCFH-DA 的终浓度为2-5 μM。探针装载的时间也可以根据情况在15-60 min 内适当进行调整。
4. 活性氧阳性对照(Rosup) 仅仅用于作为阳性对照的样品,并不是在每个样品中都需加入活性氧阳性对照。
5. 探针装载完毕并洗净残余探针后,可以进行激发波长的扫描和发射波长的扫描,以确认探针的装载情况是否良好。DCF的激发光谱和发射光谱请参考上图。
6. 尽量缩短探针装载后到测定所用的时间(刺激时间除外),以减少各种可能的误差。
7. 为了您的安全和健康,请穿实验服并戴一次性手套操作。
8. 定量的话要作标准曲线吧。先做一个不同浓度H2O2氧化DCFA荧光值,做一条标准曲线,X轴为H2O2浓度,y轴是荧光值,得出一个方程,在看你样品的荧光值即Y值是多少,对应的X值就是。
9. 有的细胞装载探针后细胞容易漂起来,洗细胞时实验组会吸走一部分细胞。所以种细胞时细胞量增加一倍,这样细胞紧密连接,贴壁比较牢,实验组的荧光值就高了。另外的H2DCFDA很敏感,工作液浓度要低一些,1-2μM就够啦,浓度太高容易有非特异性染色。这个探针很不稳定,一旦氧化了本底荧光值就会升高,最好工作液现用现配。
使用说明
1. 装载ROS 探针
1.1 原位装载探针(仅适用于贴壁细胞)
a) 细胞准备:检测前一天进行细胞铺板,确保检测时细胞数量小于5×10^5/ml。
b) 药物诱导:去除细胞培养液,加入无血清培养基稀释的药物处理,于37℃细胞培养箱内避光孵育,实际诱导时间由药物特性和细胞类型决定。
c) (可选)阳性对照:先用无血清培养基等稀释阳性对照(Rosup, 100 mM)到常用工作浓度100 μM,加入细胞,37℃避光孵育0.5~4 h,以提高活性氧水平,不同细胞类型存在差异。例如:HeLa 细胞需孵育30-60 min,MRC5 人胚胎成纤维细胞则需孵育90 min。
d) ROS 探针准备:探针装载前按照1:1000 用无血清培养液稀释DCFH-DA,使其终浓度为10 μM。
e) ROS 探针装载:吸除处理药物,加入适当体积稀释好的DCFH-DA 工作液。加入的体积需充分盖住细胞。例如:6孔板通常不少于1000 μL,对于96 孔板通常不少于100 μL。37℃细胞培养箱内避光孵育30 min。
f) 细胞清洗:用无血清培养液洗涤细胞1~2 次,以充分去除未进入细胞内的DCFH-DA。
1.2 收集细胞后装载探针(适用于贴壁细胞和悬浮细胞)
a) 细胞准备:按照标准方法培养细胞,必须保证检测用细胞状态。按照适当方法,清洗并收集足量的细胞。
b) 药物诱导:将收集好的细胞悬浮于适量稀释好的药物,于37℃细胞培养箱内避光孵育,实际诱导时间由药物特性和细胞类型决定。
c) (可选)阳性对照:先用无血清培养基稀释阳性对照(Rosup, 100 mM)到常用工作浓度100 μM,加入细胞,37℃避光孵育0.5~4 h 以提高活性氧水平,不同细胞类型存在差异。例如:HeLa 细胞需孵育30-60 min,MRC5 人胚胎成纤维细胞则需孵育90min。
d) ROS 探针准备:探针装载前,按照1:1000 用无血清培养液稀释DCFH-DA,使其终浓度为10 μM。
e) 探针装载:除去细胞内药物,离心收集细胞,加入稀释好的探针,使其细胞密度为1×10^6 ~ 2×10^7。
注意:细胞密度需根据后续的检测体系,检测方法,以及检测总量来进行调整。例如:对于流式分析,单管检测内细胞数目不少于10^4,也不可多于10^6。
f) 细胞清洗:用无血清细胞培养液洗涤细胞1-2 次,以充分去除未进入细胞内的DCFH-DA。
2. 荧光显微照相操作方法
a) 对贴壁生长细胞或活组织,可直接在荧光显微镜下观察;对悬浮生长细胞,取25-50 μL 细胞悬液滴到一张显微载玻片上,再盖上一张盖玻片。
b) 荧光显微镜下,选用FITC 滤光片观察荧光,去除背景观察荧光的变化。
3. 流式细胞分析操作方法
a) 对贴壁生长细胞,用胰酶消化制备成单细胞悬液;对悬浮生长细胞,直接收集细胞。用0.5-1 mL PBS 重悬细胞(0.5~1 x 10^5/ml)。
b) 选择流式细胞仪FL1 或BL1 通道,488nm 激发,测定530nm 的发射,细胞应可分成两个亚群:ROS 阴性细胞仅有很低的荧光强度,ROS 阳性细胞有较强的绿色荧光。
4.参数设置
使用488nm激发波长,525nm发射波长,实时或逐时间点检测刺激前后荧光的强弱。DCF的荧光光谱和FITC非常相似,可以用FITC的参数设置检测DCF。DCF的激发光谱和发射光谱参考下图。
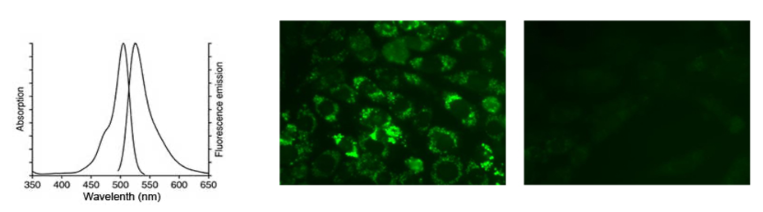
使用活性氧检测试剂盒(Reactive oxygen species assay kit)显示CHO细胞内活性氧荧光。
左图:CHO细胞用试剂盒配备的活性氧阳性对照处理;右图:正常CHO细胞。绿色荧光表明细胞活性氧急剧增加,并能显示其定位。
Product composition
|
|
Product name
|
Package
|
|
Liquid A
|
H2DCFH-DA(10mM)
|
0.1mL
|
|
Liquid B
|
Active oxygen positive control(Rosup,50mg/mL)
|
1mL
|
Product Introduction
Reactive Oxygen Species Assay Kit (Reactive Oxygen Species Assay Kit) is a kit that uses fluorescent probe H2DCFH-DA for reactive oxygen detection. H2DCFH-DA itself has no fluorescence and can freely pass through the cell membrane. After entering the cell, it can be hydrolyzed by intracellular esterase to produce DCFH. DCFH cannot penetrate the cell membrane, so that the probe is easily loaded into the cell. The reactive oxygen species in cells can oxidize non-fluorescent DCFH to produce fluorescent DCF. Detecting the fluorescence of DCF can know the level of reactive oxygen species in the cell. According to the fluorescence produced in living cells, the content and changes of reactive oxygen species can be judged. It can be directly observed with a flow cytometer or a fluorescence microscope. It is a classic method for detecting reactive oxygen species in tissues or living cells. This kit provides Rosup, a positive control reagent for active oxygen, to facilitate the detection of active oxygen. Rosup is a mixture with a concentration of 50 mg/mL. Rosup is a reactive oxygen-inducing drug. According to its fluorescence signal intensity, the true level of reactive oxygen can be analyzed.
The kit has low background, high sensitivity, wide linear range and convenient use. This kit can measure 1000 samples 1000T (96 well plate).
Precautions
1. After the probe is loaded, be sure to wash the remaining probes that have not entered the cell, otherwise it will cause a high background.
2. The positive control Rosup generally uses a concentration of 100 μM (the recommended concentration is 100-400 μM, depending on the cell type). Usually, a significant increase in reactive oxygen species can be observed 0.5-4h after stimulation. For different cells, the effect of reactive oxygen species positive control may be quite different. If no increase in active oxygen is observed within 30 minutes after stimulation, the induction time can be extended or the concentration of active oxygen positive control can be appropriately increased. If the reactive oxygen species increase too quickly, shorten the induction time or appropriately reduce the concentration of the reactive oxygen species positive control.
3. For some special cells, if the negative control cells without stimulation are found to have stronger fluorescence during the experiment, you can dilute DCFH-DA at 1:2000-1:5000 to make the final concentration of DCFH-DA 2-5 μM. The loading time of the probe can also be appropriately adjusted within 15-60 min according to the situation.
4. Active oxygen positive control (Rosup) is only used as a positive control sample, and it is not necessary to add active oxygen positive control to every sample.
5. After the probe is loaded and the remaining probes are cleaned, scan the excitation wavelength and emission wavelength to confirm whether the probe is well loaded. Please refer to the figure above for the excitation and emission spectra of DCF.
6. Try to shorten the time from probe loading to measurement (except stimulation time) to reduce all possible errors.
7. For your safety and health, please wear lab coats and disposable gloves.
8. If it is quantitative, make a standard curve. First make a different concentration of H2O2 oxidized DCFA fluorescence value, make a standard curve, the X axis is the H2O2 concentration, the y axis is the fluorescence value, and get an equation. Look at the fluorescence value of your sample, that is, the Y value, and the corresponding X value Yes.
9. After some cells are loaded with probes, the cells are easy to float up, and the experimental group will suck some of the cells when washing the cells. Therefore, the cell mass is doubled when the cells are planted, so that the cells are tightly connected and adhere to the wall more firmly, and the fluorescence value of the experimental group is higher. In addition, H2DCFDA is very sensitive. The concentration of the working solution should be lower, 1-2μM is enough. If the concentration is too high, non-specific staining may occur. This probe is very unstable. Once it is oxidized, the background fluorescence value will increase. It is best to use the working solution and prepare it.
Instructions for use
1. Load ROS probe
1.1 In-situ loading of probes (only for adherent cells)
a) Cell preparation: Plating cells the day before the test to ensure that the number of cells during the test is less than 5×10^5/ml.
b) Drug induction: Remove the cell culture medium, add the drug treatment diluted with serum-free medium, and incubate in a 37°C cell culture incubator in the dark. The actual induction time is determined by the characteristics of the drug and the cell type.
c) (Optional) Positive control: First dilute the positive control (Rosup, 100 mM) with serum-free medium, etc. to a common working concentration of 100 μM, add cells, and incubate at 37°C for 0.5 to 4 h in the dark to increase the level of reactive oxygen species , Different cell types are different. For example: HeLa cells need to be incubated for 30-60 minutes, and MRC5 human embryonic fibroblasts need to be incubated for 90 minutes.
d) ROS probe preparation: Before loading the probe, dilute DCFH-DA with serum-free culture medium at a ratio of 1:1000 to make the final concentration 10 μM.
e) ROS probe loading: aspirate the treatment drug, and add DCFH-DA working solution diluted in an appropriate volume. The added volume must cover the cells sufficiently. For example, a 6-well plate is usually no less than 1000 μL, and a 96-well plate is usually no less than 100 μL. Incubate for 30 min in a cell culture box at 37°C in the dark.
f) Cell washing: Wash the cells with serum-free culture medium 1 to 2 times to fully remove the DCFH-DA that has not entered the cells.
1.2 Load probes after collecting cells (applicable to adherent cells and suspension cells)
a) Cell preparation: Cells are cultured according to standard methods, and the cell status for testing must be guaranteed. According to the appropriate method, wash and collect enough cells.
b) Drug induction: Suspend the collected cells in an appropriate amount of diluted drug, and incubate in a 37°C cell incubator in the dark. The actual induction time is determined by the characteristics of the drug and the cell type.
c) (Optional) Positive control: First dilute the positive control (Rosup, 100 mM) with serum-free medium to a common working concentration of 100 μM, add cells, and incubate at 37°C in the dark for 0.5 to 4 hours to increase the level of reactive oxygen species. There are differences in cell types. For example: HeLa cells need to be incubated for 30-60 minutes, and MRC5 human embryonic fibroblasts need to be incubated for 90 minutes.
d) ROS probe preparation: Before loading the probe, dilute DCFH-DA with serum-free culture medium at a ratio of 1:1000 to make the final concentration 10 μM.
e) Probe loading: remove the intracellular drugs, collect the cells by centrifugation, and add the diluted probe to make the cell density 1×10^6~2×10^7.
Note: The cell density needs to be adjusted according to the subsequent detection system, detection method, and total detection volume. For example, for flow cytometry, the number of cells in a single tube is not less than 10^4 and not more than 10^6.
f) Cell washing: Wash the cells with serum-free cell culture medium 1-2 times to fully remove the DCFH-DA that has not entered the cells.
2. Fluorescence microscopy operation method
a) For adherent growth cells or living tissues, you can directly observe under a fluorescence microscope; for suspended growth cells, drop 25-50 μL of cell suspension onto a microscope slide, and then cover with a cover glass.
b) Under a fluorescence microscope, use FITC filters to observe fluorescence, and remove background to observe changes in fluorescence.
3. Flow cytometry operation method
a) For adherent growth cells, trypsinization is used to prepare a single cell suspension; for suspension growth cells, the cells are directly collected. Resuspend the cells with 0.5-1 mL PBS (0.5-1 x 10^5/ml).
b) Choose the FL1 or BL1 channel of the flow cytometer, excite at 488nm, and measure the emission at 530nm. Cells should be divided into two subgroups: ROS negative cells have only very low fluorescence intensity, and ROS positive cells have strong green fluorescence.
4. Parameter setting
Using 488nm excitation wavelength, 525nm emission wavelength, real-time or time-by-time detection of fluorescence intensity before and after stimulation. The fluorescence spectrum of DCF is very similar to FITC, and the parameters of FITC can be used to detect DCF. Refer to the figure below for the excitation and emission spectra of DCF.

Reactive oxygen species assay kit was used to display the fluorescence of reactive oxygen species in CHO cells.
Left image: CHO cells were treated with the reactive oxygen species included in the kit; right image: normal CHO cells. Green fluorescence indicates a sharp increase in cellular reactive oxygen species and can show its location.